 When venous clots break off and travel through a patient’s circulatory system, they can become trapped in the lung and block blood flow. This strains the heart’s ability to pump blood through the lungs and can ultimately lead to heart failure.
When venous clots break off and travel through a patient’s circulatory system, they can become trapped in the lung and block blood flow. This strains the heart’s ability to pump blood through the lungs and can ultimately lead to heart failure.
Traditionally, patients with pulmonary embolisms are treated overnight with systemic infusions of tissue plasminogen activator.
But, according to Dr. Victor Tapson at Cedars-Sinai Medical Center in Los Angeles, large doses of tPA are associated with side effects including intracranial bleeding and smaller doses can be safer and just as effective as the larger, conventional doses.
Data from BTG‘s (LON:BTG) Optalyse PE trial showed that the company’s drug-device combination can effectively treat pulmonary embolism with a smaller dose of drugs and in less time than the standard of care.
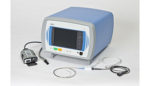
London-based BTG developed the only endovascular device that is FDA-approved to treat pulmonary embolism. The Ekos system consists of an ultrasonic device and a thrombolytic drug used to dissolve a clot.
The 101-patient Optalyse trial randomized participants to receive therapeutic anticoagulation and Ekos acoustic pulse thrombolysis therapy at different doses for different lengths of time. The 1st group received 4mg per catheter of tissue plasminogen activator over 2 hours, while the 2nd cohort received the same dose over 4 hours. The 3rd group was treated with 6mg per catheter over 6 hours and the 4th group received 12mg per catheter over 6 hours.
 All 4 groups saw a significant reduction in the main indicator of right heart strain from pulmonary embolism of 23% to 26%, which is consistent with results from previous Ekos studies when treatment was applied for up to 24 hours, according to BTG.
All 4 groups saw a significant reduction in the main indicator of right heart strain from pulmonary embolism of 23% to 26%, which is consistent with results from previous Ekos studies when treatment was applied for up to 24 hours, according to BTG.
The trial’s results also showed a low bleeding rate of 3% compared to 10% in a previous study where patients were treated with 24mg for 12 or 24 hours.
Ekos VP and general manager Matt Stupfel said the study proves that the Ekos system can “get the patient out of harm’s way quickly and it also gives the ability to reduce costs, shorten the treatment time and shorten the drug dosage.”
For Tapson, the study’s principal investigator, the recently-published data demonstrated something that he said he has instinctively known for 2 decades – that lower doses and shorter infusions make sense for patients suffering from pulmonary embolism.
“There’s no reason why you should need a 15-hour, 24-hour infusion for pulmonary embolisms,” he told Drug Delivery Business News. “I think we’ve moved the science ahead some in documenting that short infusions, lower doses are appropriate and I think this will help the design of future studies so people don’t waste their time using high doses of drug and longer infusions.”
With more clinical data from studies enrolling a larger patient population, physicians could feel confident in moving away from a 24 milligram dose of tPA, according to Tapson.
“Certainly in my mind we’ve already eliminated the 15- or 24-hour infusions,” he said.
With more clinical data for Ekos on the way later this year, Stupfel said that BTG will continue to refine its drug-device combo.
“We’re going to continue marching down the technology line to improve our technology, but also to provide technology that can treat patients faster, better, and hopefully cheaper,” he said.
