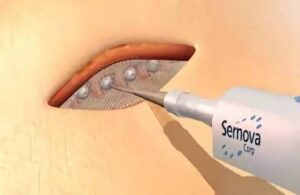
London, Ontario-based Sernova designed its proprietary Cell Pouch System as an implantable and scalable medical device. It forms a natural environment in the body for the long-term survival and function of therapeutic cells. Those cells release necessary proteins or factors missing from the body to treat chronic diseases, including insulin-dependent diabetes.
The University of Chicago Institutional Review Board (IRB) approved a protocol amendment to advance Sernova’s ongoing Phase 1/2 clinical trial. The study evaluates six patients with long-standing, insulin-dependent type 1 diabetes (T1D). Those patients have hypoglycemia unawareness prior to treatment. Sernova announced positive data from that trial in June. That includes three patients achieving insulin independence.
With no objection from the FDA, Sernova may proceed with a strategically optimized protocol reducing the time required for patient treatment. It also accelerates potential secondary endpoint efficacy achievement with more optimal dosing.
About the new protocol
The protocol amendment enables the testing of a small cohort of up to seven patients. That cohort would receive Sernova’s optimized 10-channel Cell Pouch. It provides 50% more islet capacity compared to the eight-channel Cell Pouch used to date in the T1D study. The company expects results to help guide the design of its pivotal study to support its anticipated biologics license application (BLA) to the FDA.
Enrollment in the first cohort concludes with the protocol amendment. Sernova now plans to begin enrollment in the second cohort. The company engaged a clinical trial patient recruitment partner to expedite enrollment for the second cohort.
“Sernova is committed to developing a potential ‘functional cure’ with the goal of freeing patients from the life-limiting burdens of T1D and dramatically enhancing their quality of life,” said Dr. Philip Toleikis, President and CEO. “We are pleased with the positive interim results demonstrating that the Cell Pouch System is well tolerated, reduces or eliminates the frequency of severe hypoglycemic events and continues to contribute to durable insulin independence among patients. Based on our now expanded recruitment activities and efforts, we anticipate implanting multiple patients before the end of 2022. The results from these additional patients will directly contribute to our pivotal T1D trial design and help accelerate entry into the clinic with our iPSC stem cell-derived islet technology in conjunction with our Evotec partnership that is progressing ahead of our expectations.”
Details on the second cohort in the Sernova study
Sernova’strial evaluates the safety, tolerability, and efficacy of the Cell Pouch System. Patient eligibility remains unchanged for the second cohort. That means it includes those aged 18-65 with T1D who suffer from both hypoglycemic unawareness and severe hypoglycemic episodes. They must have eligibility for donor islet transplantation.
Eligible trial patients experienced at least one episode of severe hypoglycemia in the previous 12 months. They also report an absence of stimulated C-peptide in response to a mixed meal tolerance test.
Islet transplant into the Cell Pouch takes place approximately six weeks after Cell Pouch implantation. This allows for the development of fully vascularized native tissue chambers. That helps to achieve a stable immunosuppressant regimen.
At 90 days post-islet transplant, the study assesses safety and efficacy before initiating a second islet transplant. Ninety days after that, the study evaluates safety and efficacy again. Patients remaining dependent on insulin for at least six months after the second transplant may retain eligibility for a third transplant.
The Sernova study follows patients who retain their implants for up to three years after the final islet transplant. Multiple transplanted patients from the first cohort have implant durations of more than three years. None elected to have their Cell Pouch implants removed.

