A cure for type 1 diabetes (T1D).
“I just thought it was an incredible idea,” said Toleikis, who serves as Sernova’s president and CEO. “We spent a lot of time working on it, and we’ve gotten up to this point now.”
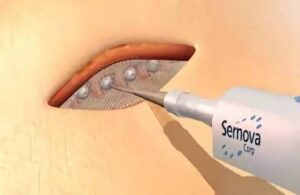
“This point” includes in-human trials for Sernova’s Cell Pouch System. London, Ontario-based Sernova designed its proprietary Cell Pouch System as an implantable and scalable medical device. It forms a natural environment in the body for the long-term survival and function of therapeutic cells. Those cells release necessary proteins or factors missing from the body to treat chronic diseases, including insulin-dependent diabetes.
About Sernova’s ongoing trials
Sernova’s ongoing Phase 1/2 clinical trial evaluates six patients with long-standing, insulin-dependent T1D.
Those patients have hypoglycemia unawareness prior to treatment. Sernova announced positive data from that trial in June. That includes three patients achieving insulin independence. Earlier this month, the company received approval to proceed with a strategically optimized protocol reducing the time required for patient treatment. It also accelerates potential secondary endpoint efficacy achievement with more optimal dosing.
Last week, Sernova implanted two patients in its second cohort with its 10-channel Cell Pouch. The 10-channel Cell Pouch has 50% more islet capacity compared to the eight-channel Cell Pouch. The first cohort of the clinical trial utilized the eight-channel pouch.
The higher dose of cells spreads the treatment out within the device chambers. Sernova believes that can increase the efficacy even further.
“We worked on our first cohort of patients and got very good safety data,” said Toleikis. “We got some efficacy data we were really happy with. But, the one thing we wanted to do was to increase the dose of cell islets going in. We also wanted to spread them out because the islets are reading the local levels of blood sugar.”
More on the Cell Pouch technology
Patients receive Sernova Cell Pouch device implant under the skin and in the belly area. The placement comes at the level of the muscle, under the fat tissue.
Toleikis explained that this offers a high level of vascularization. Plus, with the device’s flexibility, the patient doesn’t really notice it’s there because of how deep the implant is. The longest a patient has lived with the device implanted is more than three years, now.
With a donor islet from the pancreas placed in the device, Sernova observed function. Function came in the form of the C peptide biomarker measurement. C peptide is released when islets release insulin into the bloodstream. The device demonstrated strong safety and efficacy in small and large animals, leading to ongoing human trials.
Islets managed to survive and produce insulin with the Sernova Cell Pouch. It represented a massive step forward, Toleikis said, because a major problem with implanted devices like this can come from the body’s response.
“Essentially, when you put a typical device in the body, the body will just try to wall it off,” said Toleikis. “So, we developed this device to enable tissue to grow into it so it healed. That becomes a part of the body and then the body would not reject that device.”
Sernova now works with technology brought in from the University of Miami called conformal coating. The crosslinked polymer coating goes around the cells themselves.
This allows the cells to go into the device chambers, sit in the rich, vascularized tissue environment and still survive and function.
“That could eliminate the need for patients to take anti-rejection medications and significantly expand our patient population,” noted Toleikis.
What the future holds for Sernova
Sernova also has a partnership with Evotec over stem cell-derived technology. They aim to develop an implantable iPSC-based beta cell replacement therapy for the treatment of insulin-dependent diabetes. It would cover both T1D and T2D. Toleikis said the technology is “rapidly moving towards the clinic.”
Altogether, Sernova expects a “very exciting” coming year. With new data from newly enrolled patients, plus work coming through on the Evotec front, Toleikis said “great times” could be on the horizon.
Plus, Cell Pouch isn’t limited to just diabetes. The platform technology can operate with different types of cells while the device remains the same.
Toleikis said the company can make the device different sizes to accommodate different cell types, too. The company has one program for hemophilia A. Sernova believes that it can work with multiple rare diseases in that area.
Additionally, the company plans to work on an indication for patients with thyroid disease who received thyroid gland removal. Currently, the company can take the healthy tissue that remains and place it back into the device to see if they can recover a feedback loop for those patients. Toleikis said they may enter the clinic for that program in 2023.
“[We have] lots of options, but our main focus is really developing that very, very strong diabetes franchise,” said Toleikis. “We believe we are the most advanced company in the world with the data that we have and with our combination of device and therapeutic cells. This is a product we’re really excited about getting to patients.”

