Speaking at the JP Morgan Healthcare conference this week, CEO Geoff Martha highlighted the potential opportunity in diabetes.
“You’ve seen us turn around this business over the last couple of years,” Martha said. “It’s grown above our corporate average for the past six quarters and we don’t see this stopping anytime soon.”
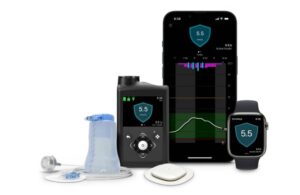
The turnaround effectively began in April 2023, when Medtronic received FDA approval for its next-generation MiniMed 780G automated insulin delivery system. Not long after, the company fully resolved a warning letter issue with the FDA. Since, the company has racked up milestones, including advancements for its InPen smart insulin pen platforms and next-generation Simplera continuous glucose monitor platform.
A major development for the company’s diabetes business, came this month when it announced a collaboration with Abbott. The partnership aims to collaborate on a system based on Abbott’s FreeStyle Libre CGMs with Medtronic’s automated insulin delivery technology (the latest generation being the MiniMed 780G) and smart insulin pen systems, such as the InPen system.
“We’re well-positioned in this $16 billion-plus market,” Martha said. “Smart dosing is expected to dominate by the end of the decade, as CGM alone is just not sufficient for this patient population. We remain the only company investing in a comprehensive ecosystem of differentiated technology for intensive insulin patients that takes on more of the work of the diabetes management and takes it off their plates.
“And these investments have brought several new innovations to market.”
What we can expect from Medtronic
Martha said “there’s more to come in the near-term,” from Medtronic, starting with the company’s CGM portfolio refresh. Add the Simplera advancements to the partnership with Abbott, and Medtronic’s CGM offerings look very different than before.
The medtech giant also anticipates several label expansions on the horizon, including into type 2 diabetes. Expansion to the type 2 population was a theme for several diabetes tech makers at the American Diabetes Association Scientific Sessions last June, and Medtronic continues to progress there.
On the company’s second-quarter earnings call in November 2024, Que Dallara, EVP and president of Medtronic Diabetes, said the company finished enrollment in a study of MiniMed 780G for type 2. Dallara set expectations for an FDA submission in the first half of calendar 2025, saying “that’s progressing very well.”
Martha labeled Medtronic “mainly a type 1 business, moving into type 2,” with the latter representing “a meaningful driver.”
“We feel great about our portfolio,” Dallara added at JPM. Especially when you juxtapose the mega trend in our space, which is the adoption of automated insulin delivery as the standard of care. Our portfolio is hand-in-glvoe with that trend.”
Dallara noted positive feedback on the CE-marked Simplera Sync and MiniMed 780G combination in Europe. She said Medtronic hopes to bring that combination to the U.S. market this calendar year, followed by developments with Abbott.
“We will be addressing the sensor form factor issues and that’s going to open up — if you look at just the access to the Abbott installed base alone — over 1 million type 1 patients and over 2 million type 2 patients,” Dallara said. “When you add up the multiplication of growth vectors, it’s hard not to be excited about the business.”
Next-gen systems on the horizon?
Last November, Martha hinted at advancements on next-generation insulin pump technology, including a patch pump system that could rival the market-leading Insulet Omnipod platform.
Medronic was set to enter the patch pump space in late 2023 with a $738 million acquisition of EOFlow. However, about six months after striking that deal, the company called it off, citing multiple breaches of their acquisition agreement.
Reports surfaced in Korea not long after the deal fell through saying that mutual interest remained between companies. However, Medtronic also said at the time that it has its own differentiated patch pump under development.
On the company’s second-quarter earnings call, Martha said the company’s patch pump programs “remain dynamic.”
“We’ve got a couple of shots on goal, there,” he said. “I don’t believe we’ve given any timing on that, other than that we’ve been investing heavily in diabetes across the entire [ecosystem]. One of our strengths is that we have the entire ecosystem, and a patch needs to be a part of that.”
At JPM, Martha reiterated that those next-generation systems are on the way.
“We have next-gen AID systems, including a patch and pump, new pump modalities, to combine with our next-gen sensor,” Martha said.
According to Diabetotech on LinkedIn, the first patch pump could hold 300 units of insulin with a 7-day wear time. It integrates Medtronic’s existing extended-wear infusion set
For the next-generation pump, Diabetotech says Medtronic expects its 800-series pump to come in at around half the size of the latest-generation 780G. The post says the company plans for a pivotal study in 2025. Potential features could include extended reservoirs and extended-wear sets, plus a brand-new Android/iOS app.
