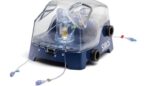
Sirtex Medical announced today that it received EU MDR certification to market its SIR-Spheres Y-90 resin microspheres and delivery systems. EU MDR authorization enables Woburn, Massachusetts-based Sirtex to kick off a European launch for its Siros delivery system. Siros offers an intuitive, visual and adaptable solution for physicians to administer SIR-Spheres to liver cancer patients. […]
Immunotherapy
Navidea names two appointments to its board of directors
Navidea Biopharmaceuticals (NYSE:NAVB) announced today that it appointed two new members to its board of directors. Thomas Forest Farb-Horch and Agnieszka Winkler joined Dublin, Ohio-based Navidea’s board, effective Oct. 7, each with a three-year term ending concurrently with the company’s 2024 annual stockholders meeting. According to a news release, Farb-Horch, a veteran of over three […]
Boston Scientific touts data from Phase 3 study of selective internal radiation therapy
Boston Scientific (NYSE:BSX) today touted data from a Phase 3 clinical trial of its TheraSphere Y-90 glass microspheres treatment. In the trial, the TheraSphere treatment successfully met both primary endpoints, including progression-free survival (PFS) and hepatic progression-free survival (hPFS) in patients with metastatic colorectal cancer (mCRC) of the liver. Marlborough, Mass.-based Boston Scientific’s TheraSphere treatment, […]
Navidea Biopharmaceuticals makes board of directors changes
Navidea Biopharmaceuticals (NYSE:NAVB) announced today that it made a number of changes on its board of directors. Dublin, Ohio–based Navidea ‘s chair of the board and director S. Kathryn Rouan, along with director Claudine Bruck, retired from those positions, effective immediately. The board appointed Alexander L. Cappello and John K. Scott, Jr., both existing members […]
TriSalus enrolls first patient in pressure-enabled drug delivery trial for treating melanoma
TriSalus Life Sciences announced today that it enrolled the first patient in its clinical study of a treatment for metastatic uveal melanoma. The PERIO-01 clinical study evaluates SD-101, an investigational toll-like receptor 9 (TLR9) agonist in adults with metastatic uveal melanoma through intravascular administration into uveal melanoma liver metastasis lesions in combination with checkpoint inhibitors […]
FDA warns on increased risk of death with Pepaxto for multiple myeloma
The FDA today issued a notice warning patients and healthcare professionals of a potentially increased risk of death with Pepaxto. Pepaxto (melphalan flufenamide), used with dexamethasone to treat patients with multiple myeloma, demonstrated an increased risk of death in the OCEAN clinical trial. The FDA required that the manufacturer, Boston-based Oncopeptides (STO:ONCO) suspend enrollment for […]
Tyme wins U.S. patent for drug delivery method for cancer treatment
Tyme Technologies (NSDQ:TYME) announced today that it received a U.S. patent for its metabolomic drug delivery platform. Bedminster, N.J.-based Tyme’s additional patent claims related to the metabolomic technology platform (U.S. Patent No. 11,058,638) involves the targeted delivery of therapeutics to cancer cells. The company’s technology fuses the tyrosine isomer racemetryosine (α-methylparatyrosine) with a second therapeutic […]
Thermo Fisher expands gene therapy portfolio
Thermo Fisher Scientific (NYSE:TMO) announced a new set of solutions to support adeno-associated viral (AAV) manufacturing. AAV, a non-pathogenic virus capable of infecting cells at various stages of growth, has become a preferred “viral vector” for delivering gene therapies, although the scalability of AAV remains a challenge. According to a news release, Thermo Fisher designed […]
Teva initiates U.S. recall of Topotecan metastatic ovarian cancer injection treatment
Teva Pharmaceuticals (NYSE:TEVA) initiated a voluntary recall of a lot of its Topotecan injection 4mg/4mL (1mg/mL) in the U.S. The voluntary recall came as a result of a complaint received from a pharmacy after a single glass particle was observed inside one vial. Following further examination of the complaint sample, two other particulates were found […]
Boston Children’s Hospital, ElevateBio enter 5-year cell, gene therapy collaboration
Boston Children’s Hospital announced today that it partnered with ElevateBio for a five-year cell and gene therapy advancement program. The collaborative agreement will seek to advance cell and gene therapy programs originating out of Boston Children’s Hospital with an introduction to Boston Children’s translational research capabilities. Cambridge, Ma.-based ElevateBio will offer Boston Children’s researchers access […]