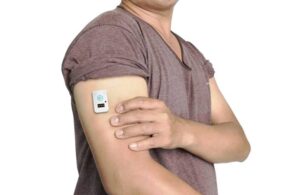
Loughborough, UK-based Nemaura Medical entered into a commercial agreement in September 2021 with MySugarWatch DuoPack Limited (MSWDL). Under the agreement, Nemaura’s non-invasive, wearable skin patch sensors paired with prescription-only medicines for type 2 diabetes. This created the MySugarWatch-branded “DuoPack.”
Nemaura Medical develops and commercializes non-invasive, wearable diagnostic devices. Its sugarBEAT and proBEAT devices provide continuous glucose monitoring (CGM) plus additional capabilities.
The combination of sensors and medicine aimed to provide a “one-stop shop” supported by a holistic care approach. This offering used MSWDL nurses and nutritionists to create a long-term motivational relationship between patient and professional.
Nemaura Medicaland MSWDL agreed to include injectable insulin for the European market as part of their agreement (as patent expiry allows). They believe as many as 4 million older patients between the UK and Europe with type 2 diabetes receive insulin.
“The inclusion of insulin in our agreement with MSWDL means patients with type 2 diabetes will have access to a CGM-guided insulin dose titration care model that is designed to reduce hypoglycemic episodes and hospital admissions while improving long-term outcomes,” said Nemaura CEO Dr. Faz Chowdhury. “Moreover, it uniquely avoids a potentially bruised, slow-to-heal, infection-prone, needle-induced skin puncture wound in older patients at the site of a CGM sensor skin application. We are eager to provide potential improvements in monitoring and lifestyle for the millions of Type 2 diabetes patients globally.”
